But some folks wonder about the environmental impacts of roadway salt and the potential to corrode cars and other vehicles. But to answer this question, one needs to start with another question. How much salt is naturally in our environment, particularly salt that reaches the ground in precipitation or by dry deposition?
The answer is going to surprise you. A lot of salt is falling out of the sky! In fact, probably more than being spread by State and local departments of transportation.
Where is it coming from? Breaking ocean waves! The ocean is salty, of course, and when waves break or when wind produces spray, the air is filled with salt water droplets. These droplets can evaporate, leaving small particles of salt in the air. Here in the Northwest, salt particles can be easily blown inland by the
prevailing westerly winds and brought back to the ground by precipitation or even dry settling (deposition).
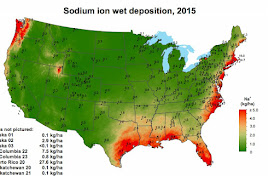
To illustrate, here is a map created by the National Atmospheric Deposition Program, based on combining observations and models, that shows the wet deposition (by precipitation) of one component of salt, sodium ions. (Remember, from your basic chemistry that salt is sodium chloride, NaCl). The red areas show large depositions (more an 4 kilograms per hectare). And remember, a kilogram (kg) is 2.2 pounds and 1 square mile is 259 hectares.
You note there is more salt deposition near coastlines and particularly stormy coastlines. That is why the Northwest is well salted but California is not. You can see the effects of the Great Salt Lake as well. Other years look very similar to 2015.
So based on such research it has been found that the Puget Sound lowlands get around 7 kg per ha per year of salt and the coast (closer to the breaking waves) receives about 30 kg per ha.
So there is considerable salt falling on the surface by natural processes, with little obvious problems for animals and plants.
OK, now let's have some fun, comparing the amount of salt falling on Seattle each year against the amount of salt resulting from roadway protection in the city.
Seattle encompasses 83.78 square miles. Each square mile is comprised of 260 ha. Thus, the city has 21,783 ha. So if 7 kg of salt falls per year per ha, then the total amount of salt reaching the ground in Seattle each year is 335,455 pounds of salt or 168 tons.
Mama mia! We have a salty city!
Let's compare this total to how much salt is used to protect the roads for one snow storm, which seems to be the typical number per winter the last few years. From a little digging, I believe that Seattle uses about 100 tons (200,000 pounds) for a single snow event--less than the 168 tons noted above. But even I am off a bit, I suspect the bottom line is reliable:
The natural precipitation of salt from the ocean over Seattle is roughly equal to the salt spread for reducing icing during a single storm.
Thus, I suspect roadway salt is not a significant environmental hazard. Furthermore, since the salt is spread over roads much of the salt goes into drains, some of which go directly to treatment plants and the Sound. This concern is further reduced by the fact that Puget Sound is a salt-water estuary, with considerable salt content (although a bit less than the ocean, 2.9% versus 3.4%).
Roadway salt, spread during heavy snow periods, does not go into drains immediately, but rather over several days, thus reducing pulses of salt. Furthermore, it is accompanied by a surge of melt water that dilutes its concentrations. As an estuary expert I consulted stated: "dilution is the solution". You've got to love sayings like that.
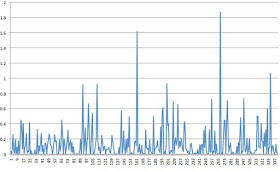
It is interesting to note that the natural deposition of salt does not fall in a uniform way, but accompanies the major storm/rain events that also bring large waves and lots of sea spray. I have confirmed this by looking at the weekly data available from the national deposition site. Here is an example showing the weekly amounts of sodium from salt at La Grande, in Pierce County from January 2010 to now. Lots of variability.
Thus, there are natural spikes in salt deposition, with only a few weeks each year providing much of the annual deposition.
I am not saying the salt on roadways is absolutely harmless, but that the risk is small compared to the alternative loss of life, injury, and economic damage. Like is about risk versus reward, costs versus benefits, and in this case the benefits of using road salt one or two times a year far outweigh the risks.
Finally, modern vehicles have far better primers and paints, and thus are less susceptible to salt-associated corrosion.
Clearly, the environment implications far salt would be far greater for cities with a lot of snow that require many salt applications over the entire winter--such as Chicago or Buffalo. Thus, Mayor Murray and the folks at SDOT can probably sleep soundly knowing the Seattle's occasional salt spreading is not undermining the environmental quality of the city. A previous Seattle mayor learned the hard way the dangers of not using salt.
Acknowledgments: I received guidance from Joel Thornton and Dan Jaffe, experts in atmospheric chemistry at the University of Washington.





I think you left one bit out of your calculation.
ReplyDeleteRoad salt is distributed over a much smaller bit of Seattle's land area than natural salt. It's just distributed on road. I would expect the concentration of road salt hitting cars is MUCH higher than the salt distributed naturally. Same goes for the road's drainage paths.
Seems dubious to compare salt amounts applied to roads and highways against sea salt sprinkled diffusely throughput the region via rain. Obviously the former will have a much more direct effect on adjacent streams and rivers (and car underbodies).
ReplyDeleteBut Cliff.
ReplyDeleteI'm someone who buys older vehicles and I've had to draw some hard and fast rules when it comes to cars and where they have spent the bulk of their life on US Roads. I understand your numbers. Isn't the worry about the intensity during particular times rather than an even distribution? After a snow storm like the one we had, the salinity levels in local streams will rise to higher levels than seen the rest of the year and that temporary higher level does damage not seen when you even it out.
As for cars, I drive my Xterra on the beach at Long Beach once a year and each time I leave the beach I drive to a car wash and give it a complete rinse; top to bottom. I can't do that after my commute each day when salt is applied to the roads and I worry that Northwest Cars will start to resemble the rust-buckets of the mid-west and east. I'm not arguing not to salt, but I don't think taking a yearly average is the right way to look at the numbers.
Charles
Doubling the amount of salt, should make more difference at all!!
ReplyDeleteNaCL is common Table Salt, but is it what the city of Seattle uses on the roads?
ReplyDeleteI know that the ice melt I used recently listed Mg as the metal side of the molecule.
I had a typo in the original
ReplyDelete1 Ha is 100 ares, not acres.
So 1 ha is about 2.5 acres
That typo did not effect the calculation, which is correct
John Renehan....my point is that the natural system is exposed to salt already. Furthermore, most of the road salt in Seattle never gets into the surrounding terrain, but washes into drains and out to the sewage plants and salty Puget Sound.
Others...the fact that cars are MUCH better protected from salt is obvious to those back East where massive amounts of salt are used. My friends there tell me that the rust and corrosion commonly seen in 70s cars is now rare. But it is good to wash a car off after a snow event...particularly the underbody
I don't think most storm drains - where road runoff goes - lead to treatment plants. In fact, many have labels on them that say "Only Rain Down the Drain" or "Flows directly to Bay" or some such. That is why getting copper out of brake pads and drips from your vehicle fixed are so important to the health of Puget Sound.
ReplyDeleteSalt data from past storms is no longer applicable. In the past SDOT only used ant-icing fluid with occasional post-snow treatment of problem areas with granular salt. I could easily see this adding up to 100 ton/event.
ReplyDeleteStarting this year they now spew countless truckloads of straight rock salt even for non-snow freeze warnings, I guarantee the value far exceeds 100 tons per actual snow day.
I moved to Portland over five years ago, but my car sat outside on the streets of Chicago for over fourteen years - with tons of sludge, dirt and mounds of salt encrusting it's every fiber of being. The result? The undercarriage certainly looks like it's from Chicago (with no infrastructure damage), but the rest of the car is fine. Those who are constantly worrying about their precious vehicles somehow being ruined by one or two road applications per year of salt (if that much), are being hysterical. There is no evidence to suggest that these fears have any founding in reality.
ReplyDeleteDr. M,
ReplyDeleteThe issue, of course, is diffuse deposition vs. point or line-source deposition. Mother Nature does the former, humans do the latter, either at specific points (eg, Snoqualmie Pass) or lines (eg, I5). Salinity of runoff near point or line-sources will still be thousands of times higher than natural salinity, as roadside birches and other trees can testify.
-Tim M.
Using your numbers...... a massive amount of salt distributed in one week will certainly have a very different effect on the ecology of a system than that same amount of salt distributed in small episodes over a year.
ReplyDeleteYour evaluation when properly considered is terrifying. We save a few car wrecks and a few (thousand?) hours at huge economic and environmental cost; we already know that. But Seattle should immediately assess the effects on our ecosystem of such an extreme increase in salt, discontinue the practice until ecological impacts are better understood.
Thanks for comparing the numbers, sincerely. But you may underestimate the importance of these changes in chemistry on the biology and ecology of an area already under stress.
Tim and Tim,
ReplyDeleteI don't think anything is terrifying...that is my point. There is already a lot of salt in the environment...salt that is mainly isolated into a few events each year in the natural system. Tim...nature does have line-source concentration...the streams. On the other hand, humans concentrate the source in exactly the place that it is least likely to spread to the exterior environment...roads, which generally are drained to the Sound. It is unlikely that the salty sound is damaged by the once or twice a year effluent of diluted salt from highway protection. Tim....regarding car wrecks...what are human lives worth? Would you be willing to sacrifice the life of your child, spouse, or neighbor? And the economic implications are huge. Seattle was crippled for weeks in Dec. 2008 due to ice. Cost had to be in the hundreds of millions of dollars of lost productivity...cliff
On a side note, Cliff, the Oroville Reservoir is currently having some serious problems. They have ordered the evacuation of Oroville. I'll keep my fingers crossed that it works out OK.
ReplyDeleteThanks for doing the calculations Cliff. A back of the envelop calculation suggests the amount of salt used by City is equivalent to 0.0000000001% of the salt in Puget Sound and 0,00001% of the salt that moves in and out of the Sound every day with the tides. So this a is non-problem for Puget Sound. We have greater problems to worry about.
ReplyDeleteSo, yes the salt may affect your car body, though not so much now as much of the body work is plastic. And yes, it may affect some urban soils planted with invasive plants like blue grass lawns or pansies and primroses and daffodils or azaleas or rhoddies. But Seattle storm water drainage contains more microplastics and particles than salt: and those come from your tires, your laundry and you riding a diesel bus or even using a Prius as its tires wear out as quickly as a Lexus SUV.
Cliff, if you check with your UW colleagues in fisheries science, I think you will find there is, in fact, a great deal of concern about the effect of sudden flushes of salt-laden water into the near-shore aquatic environment via the storm drain system.
ReplyDeleteThese near-shore areas have reduced salinity in winter due to rainwater runoff. They are important nurseries for a wide variety of marine life, including young salmon. The massive flush of high-salinity runoff from salt-treated roads is deadly to sensitive marine organisms that are adapted to the lower salinity of the brackish near-shore waters.
Great discussion. It's critical to consider that salt goes directly in the street in one big storm, while the atmospheric salt is dispersed over space and time. Also, 100% of road salt is in impervious surface. So picking numbers out if the air, if 168 tins of salt arrive in, let's say, 20 inches of rain, and 75% runs off instead of percolating, that's about 5 tons of salt running off in a 1 in rain event. If all the snow events represent 2 in of precious a year (1 feet of snow by the old 12:1 ratio), then) then the salt runoff is 50 tons per 1 in of precipitation. That's 800% more salt in the runoff. Yes, some goes to the bay and some to treatment plants, but some goes into tributary streams like Piper Creek or Mercer Creek. And these are streams showing major problems with salmon survival (the reason for the copper brake pad ban). Therefore, maybe alernatives to salt should be considered for streets that drain to salmon streams.
ReplyDeleteI'm for using salt when it matters but I find your comparisons between natural salt and highway salt to be absurd.
ReplyDeleteMichaeal DelMarco...it does not help the conservation to say something is absurd, without telling why it is absurd.
ReplyDeleteSailor and Earthwater---can you point to a SINGLE study that show that salt from a once or twice a year snowstorm has any impacts on marine life in puget sound. LOTS of studies on other chemicals (like motor oil, tire particles, drugs, fertilizer). But I could not find ONE on salt. We can not deal with speculation, but facts. Tell me the studies...cliff
Here's an informative review, admittedly from Maryland where I suspect road salt applications to be much heavier. To quote,
ReplyDelete"Salinization appears to alter the ecological condition of freshwater stream ecosystems in many places including Maryland. A preliminary examination of data collected by the Maryland Biological Stream Survey indicated that benthic macroinvertebrates, fish, salamander, and freshwater mussel richness respond negatively to elevated chloride concentrations."
http://www.mde.state.md.us/programs/Marylander/Documents/2013_Stranko_Road_Salt_(final)_TMF_edits.pdf
Cliff, from the map you included, it appears that the Puget Sound lowlands are getting only about half as much salt as you used in your calculation---maybe 3-4 kg/ha. Heading east from the Sound, the lowlands are in a salt minima, then the peak happens in the mountains. Presumably this is due to the increased precipitation as one climbs out of the Puget Sound rainshadow?
ReplyDeleteInteresting to think that our Cascade forests are getting more sea salt than folks down in Seattle. (Maybe those in immediate proximity to the water--like a mile or two--do get more, and it's just not visible on the map.)
Haunma...remember the map shows only sodium, you have to add the chloride to get the total mass.
ReplyDeletePatrick... there is MUCH more use of salt in Maryland. Really can't compare to what happens here.. In addition, there are not many rivers and creeks next to roads that are exposed to salt in the city. This is not rural Maryland--we are talking about major roads such as Aurora Ave, downtown major streets, and the like.....cliff
Cliff, that's true but it certainly indicates that increased salinity can have negative ecological consequences.
ReplyDeleteOrganisms can only survive/thrive within certain environmental parameters, salinity being one of them. If your analysis is correct, ice mitigation with road salt has nearly doubled the environmental incidence of salt. Additionally, storm drains in Seattle do outfall into fresh water including Lake Washington and Lake Union, as can be seen on this map. http://gisrevprxy.seattle.gov/wab_ext/DSOResearch_Ext/
Given the lack of targeted research into this issue (specifically in Seattle) I don't believe it can be said the benefits of road salt outweigh the costs. In fact, I would argue the ecosystem services provided by a healthy and functional natural environment FAR outweigh the losses associated with icy roads, in both economic terms and in lost life years.
It never fails - Cliff posits an educated guessetimate on the impacts on a few saltings per year, then immediately the wackos come screeching out of the woodwork. Ladies and gentlemen, he's merely expressing an opinion, if you don't agree then make a coherent argument, instead of calling him names and making insinuations regarding his credibility.
ReplyDeleteAnd my point was not marine life in Puget Sound, my point was the effect of road salt on storm water entering salmon streams tributary to the Sound.
ReplyDeletePatrick and Earthwater...where is the evidence that there is any influence of one or twice a year use of salt on the natural environment? Particularly compared to the other massive and daily degradations (e.g., road oil)? I have checked through the literature and could find nothing. Some storm drains do put effluent into Lake Washington, but these are generally not salmon streams, not is there evidence that the one a year use of salt would have any negative influence...cliff
ReplyDeleteOur relatively salt-laden mountain snow is one reason (other than the temperature, which is the main reason) our snow quality is worse in these parts relative to inland mountains.
ReplyDeleteCliff, I can't point to specific studies regarding the situation in Seattle, however based on evidence from other localities and the self evident fact of road salt's alteration of the chemical environment (how ever small that effect may be) it seems prudent to examine this a little more closely before offering a full-throated endorsement of road salt based on its merits (especially since this practice does seem to be increasing). Above a certain level it is certainly not innocuous and ecological systems are so complex its real effects may not be readily obvious.
ReplyDeleteAs to your point of other pollutants (metals from gasoline additives, oil, dirt/sand, even residue from tire wear) in the runoff being more problematic than road salt, I suspect you are absolutely correct.
Anyways, love your blog! -Patrick
Some interesting opinions of road salt impacts, or ‘guesstimates’ as they have been described. Like you I prefer to rely on data and analysis rather than assumptions. We have been recording temperature and conductivity on a one-minute time step over several months in several local streams, so we have data. Conductivity indirectly measures dissolved ions in water so is an excellent surrogate for monitoring salt inputs. Our background conductivity for streams is around 200 siemens/cm, which nationally is a relatively low reading. What we observe during storm events, when most of the ocean derived salt in the map you provide would be moved inland, is a significant reduction in conductivity, commonly <50 siemens/cm and not an increase.
ReplyDeleteWhile some of our streams do directly discharge to Puget Sound, most do not. In our urban environment road run-off is directed directly and rapidly to our streams. Road salt applications are not done on an areal basis, but are applied to the road surface, which I thinks makes a comparison with areal depositions rates far too conservative to use as an estimate of potential impacts.
What we have recorded this winter during wash-off events after salt applications is a rapid conductivity increases of up to two orders of magnitude in less than an hour with elevated conductivity for up to a day and half depending on the rain or snow event. After this wash-off spike, conductivities return to background levels.
Road salt application is implicated in environmental impacts in the scientific literature. Locally, we observe very low BIBI scores indicating poor macroinvertebrate habitat in our local streams, and salmonid number in these streams are abysmal. While salmonid eggs are resilient to salt, sac fry are more susceptible.
While I do not suggest application of road salt is the major cause of poor local stream quality, I think the application of road salt, particularly the amounts we have seen on Seattle streets this winter are a likely to have a negative impact on our already impacted streams.
People find the strangest things to argue about..
ReplyDeleteIn my eyes, there's no environment to protect anywhere near a city like Seattle or Portland. Every drop of water runoff coming from Seattle is poisoned with far worse things than salt. If anything the salt may help sterilize the nastyness that runs out of these cities..
If salt helps clear the roads and keep the people safe, then it should be an easy decision.
Thanks, Cliff. A question about your map. You say "...there is more salt deposition near coastlines and particularly stormy coastlines. That is why the Northwest is well salted but California is not." However, your map is dated 2015, the peak of drought in California, with fewer storms in that region. California, particularly northern and central CA, can have huge wave action in stormy years, equaling or exceeding the Pacific Northwest. I suspect natural salt deposition during winter 2016-17 will be significantly higher in CA than your map shows. So isn't using one year's map a bit misleading? More data are needed to account for regional and temporal variability.
ReplyDeleteHerringMan,
ReplyDeleteNot misleading. Other years look very similar to 2015. If you want to check that, go to the national deposition program website....you can view them there..cliff
Working in the automotive business I can spot a Midwest or east coast vehicle in a split second once that car is on the hoist. You're right Cliff, primers and paint have come a long way but that only protects the sheet metal and body. The primary damage to vehicles from salt is every other piece of metal under a vehicle and on or around the entire drivetrain. Exhaust, suspension, brake components, frame, fasteners, mounts, hangers, u-joints and everything else that isn't rubber or plastic. And these aren't old cars, I'm talking noticeable damage in a few years and severe damage in a decade or less when a car comes from a harsh winter climate. It's sad and it gets very expensive to the consumer for extra parts and time to repair a vehicle corroded by salt.
ReplyDeleteAnd with more and more people moving around the country we see a lot more of these vehicles in our shop here in the Seattle area. So I highly recommend always having a used vehicle inspected BEFORE you buy it to be sure it has not been ruined by salt or has any other unforeseen issues.